钯催化烯丙醇的不对称迁移烷基化反应
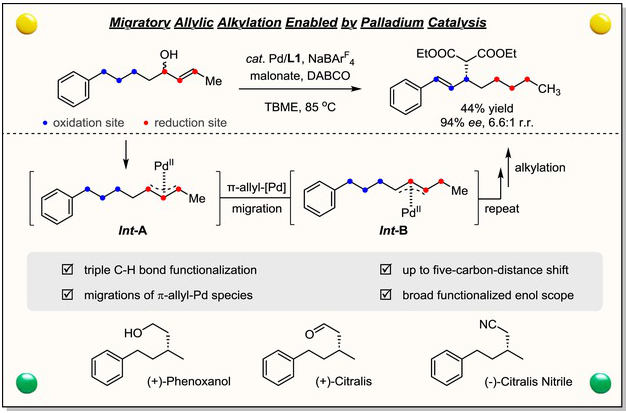
近日,复旦大学朱灿团队研究了钯催化烯丙醇的不对称迁移烷基化反应。相关论文发表在2025年12月25日出版的《中国化学》杂志上。
无导向基团(DG)参与的C(sp3)-H键对映选择性官能化反应已成为合成化学与药物化学中后期结构多样化的有力工具。
研究组开发了一种通过钯催化实现1,2-烯醇不对称迁移烯丙基取代的三重C–H键对映选择性官能化方法。该迁移烯丙基取代策略具有优异的普适性,能在精确调控化学、区域和对映选择性的条件下,兼容多种亲电试剂和亲核试剂。此迁移烷基化反应为氧化还原中性过程:三个C(sp3)–H键被氧化以实现烷基化官能化,而原始烯醇单元则同时被还原。机理研究表明,每次一碳迁移均包含连续的β-H消除与迁移插入步骤,通过二烯-钯络合物中间体形成新的π-烯丙基钯物种。该方法已成功应用于生物活性物质(+)-Phenoxanol、(+)-Citralis及(−)-Citralis Nitrile的合成。
附:英文原文
Title: Palladium-Catalyzed Asymmetric Migratory Allylic Alkylation of Allylic Alcohols
Author: Jun Zhang, Dan Zhao, Can Zhu
Issue&Volume: 2025-12-25
Abstract: Directing-group (DG)-free enantioselective functionalization of C(sp3)–H bond has emerged as a powerful tool for the late-stage diversification in synthetic and medicinal chemistry. Herein, we have developed an enantioselective triple C–H bond functionalization method via asymmetric migratory allylic substitution of 1,2-enols enabled by palladium catalysis. The robust nature of the migratory allylic substitution strategy is reflected by a broad scope of both electrophiles and nucleophiles with the control of chemo-, regio- and enantioselectivity. This migratory alkylation method is redox-neutral, with three C(sp3)–H bonds being oxidized for the alkylative functionalization and the original enol unit being reduced simultaneously. Mechanistic studies suggest that each one-carbon migration consists of the sequential β-H elimination and migratory insertion to form a new π-allylpalladium species with the intermediacy of the diene-palladium complex. This method was successfully applied for the synthesis of biologically active substances, (+)-Phenoxanol, (+)-Citralis, and ()-Citralis Nitrile.
DOI: 10.1002/cjoc.70423
Source: https://onlinelibrary.wiley.com/doi/10.1002/cjoc.70423



